Abstract
KC and ML are co-first authors.
Background:
Follicular lymphoma (FL) is the most common indolent B-cell lymphoma and has a relapsing and remitting course with risk of transformation to aggressive disease. Long-term toxicity can accumulate with repeated exposure to palliative cytotoxic chemotherapy. Rationally targeted agents have demonstrated disease control with limited toxicity in hematologic malignancies, representing a potential chemotherapy-sparing option.
BCL2 rearrangements leading to overexpression are nearly universal in FL, but BCL2 inhibition monotherapy has disappointing efficacy in FL. Potential reasons include known resistance mechanisms, such as MCL-1 overexpression, or redundant survival pathways. Founder mutations in FL often involve epigenetic regulation, and BCL2 rearrangements are necessary but not sufficient for FL lymphomagenesis. In patients with FL, abnormal DNA methylation programming cooperates with somatic mutations to drive lymphomagenesis. The resulting epigenetic deregulation may be targetable by hypomethylating agents, including azacitadine (AZA). There are no published studies of venetoclax and AZA in preclinical models of FL, but there are extensive preclinical data in acute myeloid leukemia. AZA is an epigenetic modulator with synergistic anti-leukemic effect when paired with venetoclax. Treatment with AZA results in MCL-1 downregulation, which may abrogate FL resistance to BCL2 inhibition. AZA also induces re-expression of CD20 and re-sensitizes FL patients to anti-CD20 therapy. In lymphoma xenograft models, the combination of obinutuzumab and venetoclax causes more tumor growth inhibition compared to rituximab with venetoclax, possibly from more potent direct cytotoxicity.
These preclinical studies suggest that epigenetic modulation of FL cells with AZA may increase the sensitivity to BCL2 inhibition and anti-CD20 therapy. The foundational position of epigenetic dysregulation in the clonal evolution of FL suggests the optimal time for this strategy may be early in the course of the disease. We hypothesize that a safe and tolerable dose of venetoclax, CC-486 (oral AZA), and obinutuzumab can be found with treatment efficacy equal to or better than other non-chemotherapy agents in the frontline treatment of FL. We present a dose-finding and efficacy trial of venetoclax, CC-486, and obinutuzumab in minimally pre-treated FL patients.
Study Design:
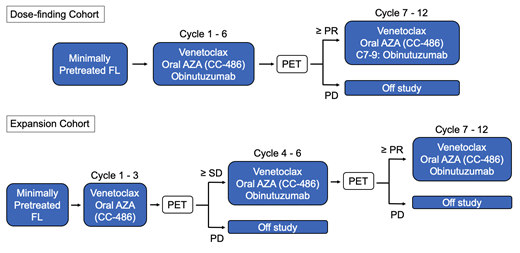
This is a single arm, multi-center phase I/II clinical trial (Figure 1). The dose finding phase is a 3+3 design with 3 escalating dose levels and two optional de-escalation levels (12-18 patients). Venetoclax (400-800mg) will be given days 1-28 of each 28 day cycle; CC-486 (150-200mg) will be given days 1-14; and obinutuzumab will be given at a fixed dose of 1000mg on days 1, 8, and 15 of cycle 1 and then day 1 of each subsequent cycle. Venetoclax and CC-486 will be given up to 12 cycles and obinutuzumab for a total of 9 cycles. Once the recommended phase 2 dose (RP2D) is identified, the study will proceed to a Simon two-stage expansion cohort. In this cohort, a three month "window" of the doublet venetoclax and CC-486 prior to introduction of obinutuzumab at cycle 4 will allow an assessment of activity of the two-drug combination. Obinutuzumab is given on days 1, 8, and 15 of cycle 4, and day 1 of each subsequent cycle. Key inclusion criteria are: age ≥ 18, ECOG ≤2; adequate kidney/liver function; and grade 1-3a FL with an indication for treatment who are either untreated or have ≤2 courses (16 doses) of lifetime anti-CD20 therapy. Patients with clinical or histologic evidence of FL transformation are excluded. Up to 32 patients will be enrolled to test the null hypothesis of a 30% CR rate against a 50% alternative (80% power, one-sided alpha=0.10).
The primary objectives are the RP2D and safety based on adverse events for the dose-finding cohort, and the CR rate by PET in the expansion cohort. Secondary objectives include progression free survival, overall survival, effect of 3 cycles of venetoclax/CC-486 in the window design of the expansion cohort, and CR rate at 30 months post-treatment with PET. Exploratory objectives include pre-treatment mutation biomarkers, minimal residual disease by circulating tumor DNA, and circulating cell-free DNA methylation patterns. This trial design was reviewed and revised at ASH CRTI. Recruitment is ongoing and this trial is registered with ClinicalTrials.gov: NCT04722601.
Kline: Kite/Gilead: Speakers Bureau; Seagen: Membership on an entity's Board of Directors or advisory committees; Regeneron: Membership on an entity's Board of Directors or advisory committees; Merck: Consultancy, Research Funding; Verastem: Research Funding; SecuraBio: Membership on an entity's Board of Directors or advisory committees; Karyopharm: Membership on an entity's Board of Directors or advisory committees; Morphosys: Consultancy, Membership on an entity's Board of Directors or advisory committees. Smith: Celgene, Genetech, AbbVie: Consultancy; Alexion, AstraZeneca Rare Disease: Other: Study investigator.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal